Class 10 Science Chapter 1 Notes Chemical Reactions and Equations We have provided class 10 science revision notes for Chapter 1 Chemical Reactions and Equations these notes give you a clear and in-depth explanation of the chapter so that class 10 students can easily understand the chapter theory concept.
| Class | Class 10 |
| Subject | Science |
| Chapter | Chapter 1 |
| Chapter Name | Chemical Reactions and Equations |
| Category | CBSE Notes |
Class 10 Science Chapter 1 Notes Chemical Reactions and Equations This note contains CBSE Key Notes, CBSE Revision Notes, and diagrams of the complete Chapter 1 Chemical Reactions and Equations of Science class 10, To score good marks in the board exam it is necessary to revise these notes, Here notes are given with detailed explanation so that it helps students to understand the chapter in a better way. Chemical Reactions and Equations Notes Those Students who are preparing for their Class 10 Board exams must go through NCERT notes for Class 10 Science Chapter 1 Notes Chemical Reactions and Equations Notes.
Class 10 Science Chapter 1 Notes Chemical Reactions and Equations
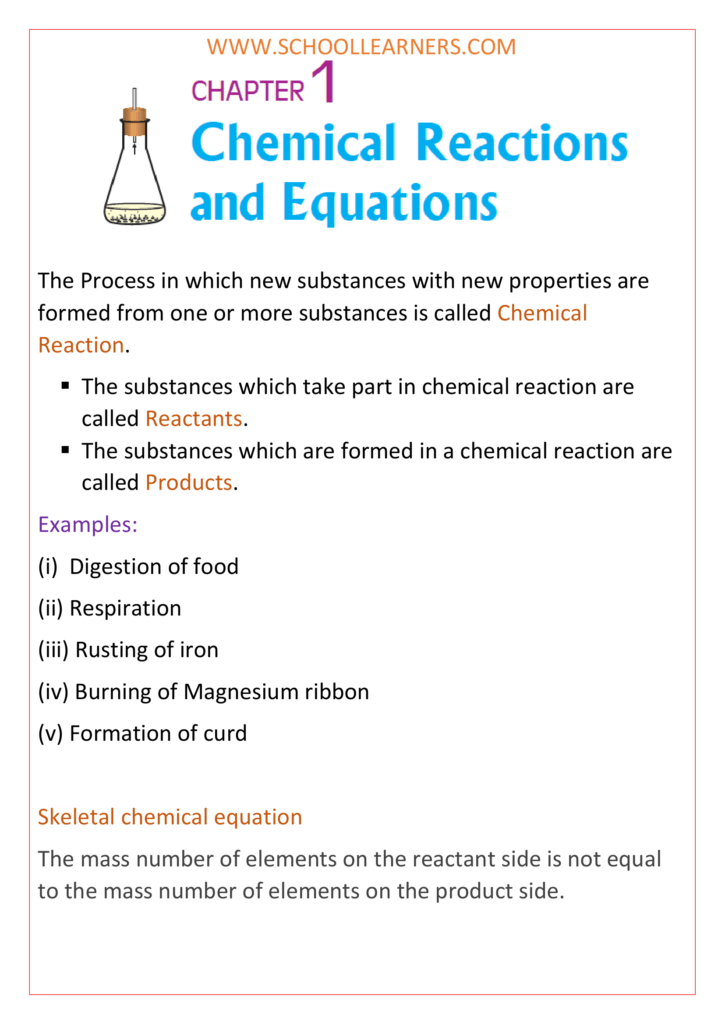

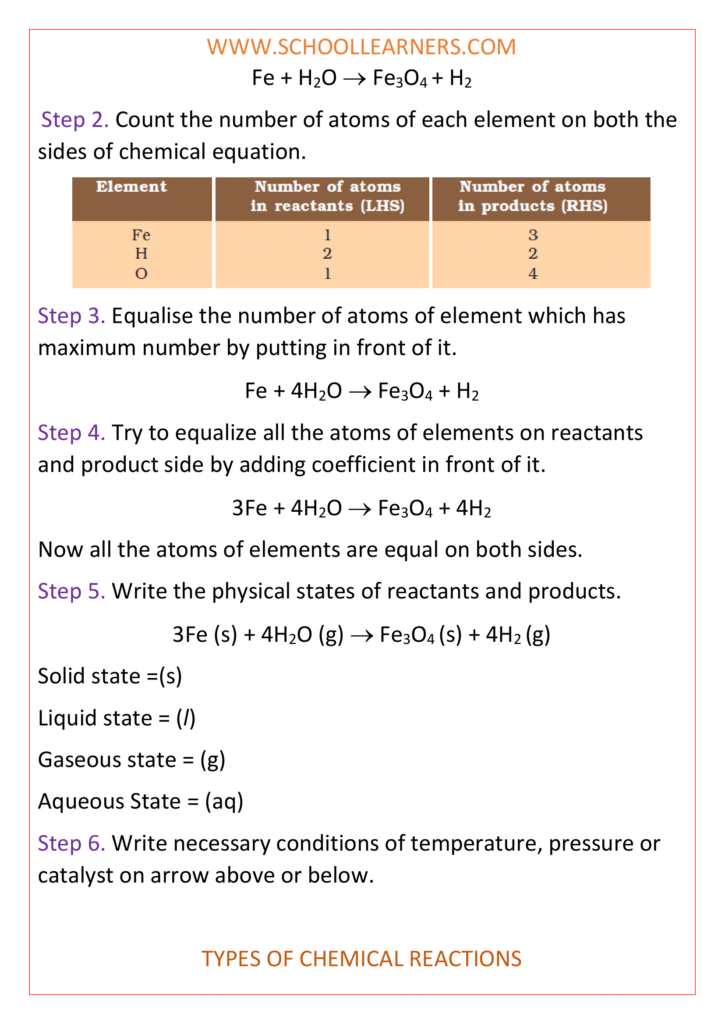

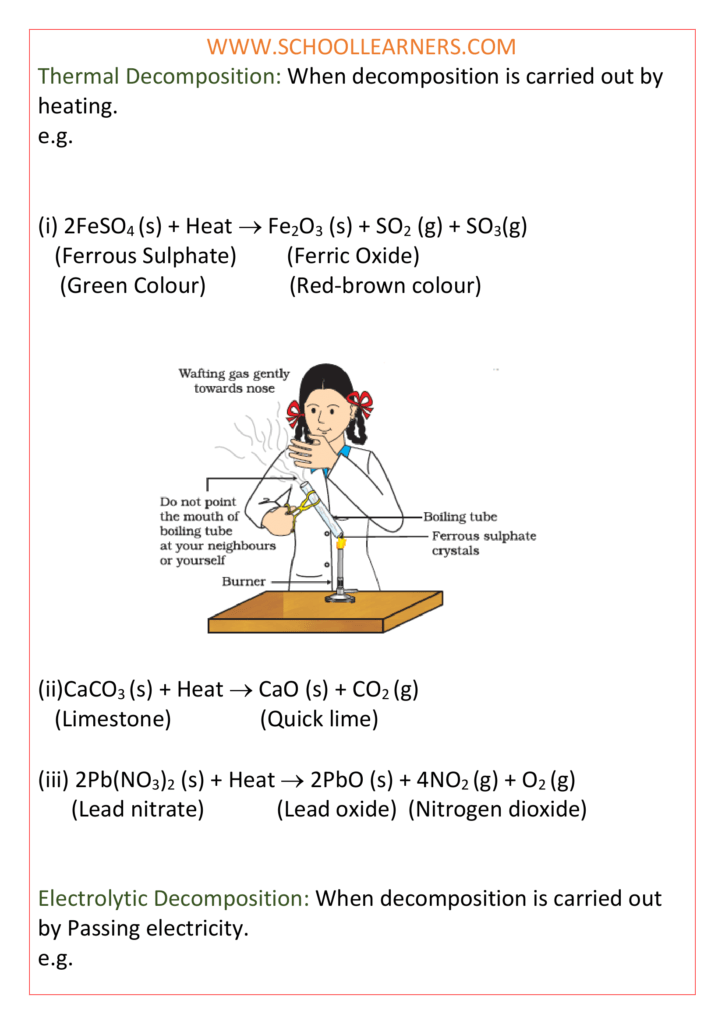
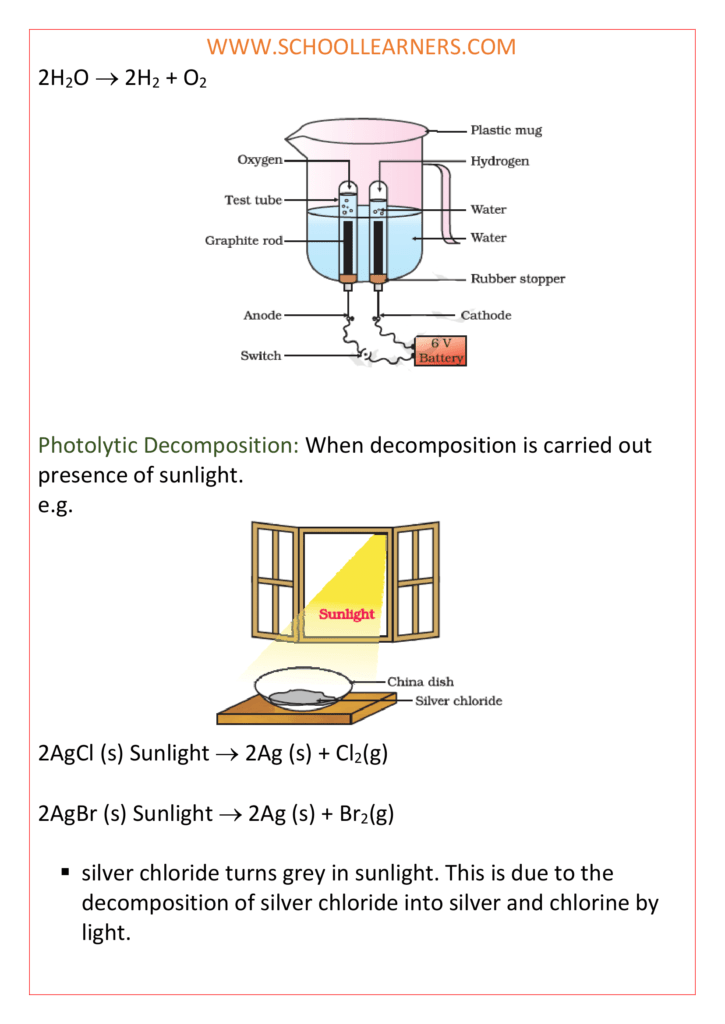



Class 10 science notes
- Chapter 1 – Chemical Reactions and Equations
- Chapter 2 – Acids, Bases, and Salts
- Chapter 3 – Metals and Non-metals
- Chapter 4 – Carbon and Its Compounds
- Chapter 5 – Periodic Classification of Elements
- Chapter 6 – Life Processes
- Chapter 7 – Control and Coordination
- Chapter 8 – How do Organisms Reproduce?
- Chapter 9 – Heredity and Evolution
- Chapter 10 – Light Reflection and Refraction
- Chapter 11 – Human Eye and Colourful World
- Chapter 12 – Electricity
- Chapter 13 – Magnetic Effects of Electric Current
- Chapter 14 – Sources of Energy
- Chapter 15 – Our Environment
- Chapter 16 – Management of Natural Resources
schoollearners.com helps you to get a good education at no cost here we provide Ncert Solutions, CBSE syllabus, and free study material also you will get access to our mentor who helps you to solve any class-related doubts, NCERT Book notes for Class 10 Science Chapter 1 Chemical Reactions and Equations Notes.
Class 10 Chemical Reactions and Equations Notes
Chemical Reactions and Equations Notes You can also Download NCERT Class 10 Science Chapter 1 Notes Chemical Reactions and Equations in Pdf form this helps you to revise the complete chapter and also help you to get score more marks in your examinations Learn more about chemical reactions and equations by exploring CBSE Notes for Class 10 Science Chapter 1.
Class 10 students can also find Ncert Intext Questions, Ncert Exercise Class 10 Science Chapter 1 Chemical Reactions and Equations Ncert Solutions will be very helpful to the class 10 students to solve their Homework and Assignments. Students can also download NCERT Solutions for Class 10 Science Chapter 1 Notes Chemical Reactions and Equations in PDF form to access them in offline mode.
Class 10 Chemistry Chapter 1 notes By Doing good practice you will easily get full marks on your board exams, the revision notes of this chapter are compulsory according to the examination point of view. Now you can learn the fundamentals of Science in CBSE Class 10 in an easy way These CBSE notes are comprehensive and detailed yet concise enough to glance through for exam preparation.
schoollearners.com 10 Science Notes Chapter 1 Chemical Reactions and Equations Pdf download, Class 10 Science Notes for Quick Revision. we have given NCERT Class 10 Science Notes Chapter 1 Chemical Reactions and Equations.
we are provided all the necessary information regarding Class 10 Science Chapter 1 Notes Chemical Reactions and Equations Notes and we hope this detailed NCERT Note is helpful. Students can also check out Ncert Solutions, CBSE Syllabus, and CBSE Sample Papers at schoollearners.com for free also you can connect with us on Whatsapp.