Class 10 Science Chapter 2 Notes Acids, Bases and Salts
ACIDS:
- These are the substances which have sour taste.
- They turn blue litmus solution red.
- They give H+ ions in aqueous solution.
- The term ‘acid’ has been derived from the Latin word, acidus, which means sour.
Strong Acids: HCI, H₂SO₄, HNO3
Weak Acids: CH3COOH, Oxalic acid, Lactic acid
Concentrated Acid: Having more amount of acid + less amount of water
Dilute Acid: Having more amount of water + less amount of acid
BASES:
- These are the substances which are bitter in taste and soapy in touch.
- They turn red litmus solution blue.
- They give OH- ions in aqueous solution.
Strong Bases: NaOH, KOH, Ca(OH)2
Weak Bases: NH4OH
Alkalis: These are bases which are soluble in water [NaOH, KOH, Ca(OH)2].
SALTS:
These are the compounds formed from reaction of acid and base.
Example: NaCl, KCI.
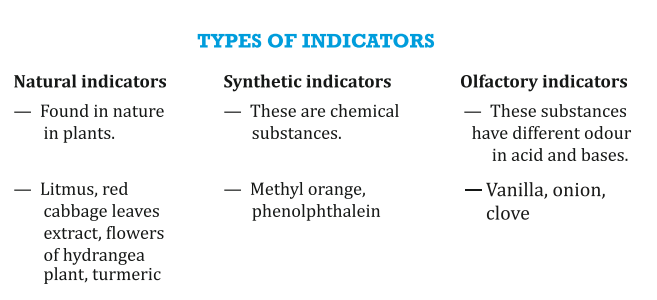
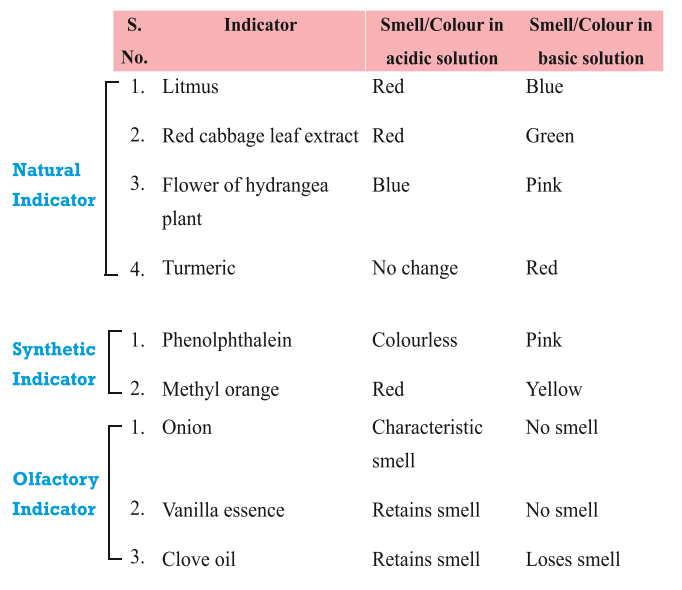
INDICATORS:
These are the substances which change their colour/smell in different types of substances.
Class 10 science notes
- Chapter 1 – Chemical Reactions and Equations
- Chapter 2 – Acids, Bases and Salts
- Chapter 3 – Metals and Non-metals
- Chapter 4 – Carbon and Its Compounds
- Chapter 5 – Periodic Classification of Elements
- Chapter 6 – Life Processes
- Chapter 7 – Control and Coordination
- Chapter 8 – How do Organisms Reproduce?
- Chapter 9 – Heredity and Evolution
- Chapter 10 – Light Reflection and Refraction
- Chapter 11 – Human Eye and Colourful World
- Chapter 12 – Electricity
- Chapter 13 – Magnetic Effects of Electric Current
- Chapter 14 – Sources of Energy
- Chapter 15 – Our Environment
- Chapter 16 – Management of Natural Resources
